XP Power Technical Director, Gary Bocock
Many medical products may come into contact with the patient or operator during normal use, or may require sensors or devices to be applied directly to the patient to perform their functions. These “applied parts” must be properly insulated from any power and ground to prevent current flow and patient harm.
The definitions of “applied part” and “medical device” are given in the 3rd edition of the medical standard IEC 60601-1, published in 2005. This standard has been adopted all over the world, for example, the European Union adopts EN 60601-1:2006/A1:2013/A12:2014, and the US adopts ANSI/AAMI ES60601-1:A1:2012, C1:2009/(R)2012 and A2: 2010/(R)2012.
This standard defines three categories of applied parts with increasing levels of risk:
• Class B (Body): Normally non-conductive and groundable applied parts.
• Type BF (Body Floating): Applied parts that are electrically connected to the patient must float and be insulated from ground. This classification excludes applied parts that are in direct contact with the heart.
• Type CF (Cardiac Float): Applied parts for direct cardiac connection. This means connections to the patient’s heart, including venous connections during dialysis. These applied parts must float and be insulated from ground.
MOOPs and MOOPs
Prevents applied parts from causing electrical shock to the patient through means of protection (MOPs) that limit hazardous voltages, currents and energy. For example, an appropriate connection to protective earth or “basic” insulation provides 1 MOP and reinforced insulation provides 2 MOP. Depending on the context, either Operator Protection Modes (MOOPs) or Patient Protection Modes (MOPPs) are defined.
For BF or CF connections, 2 x MOPP is required for AC power primary to secondary, 1 x MOPP for primary power to ground, and 1 x MOPP for output to ground. Table 1 shows the creepage and clearance required to achieve this at a “system voltage” of 250VAC and the required test voltage.
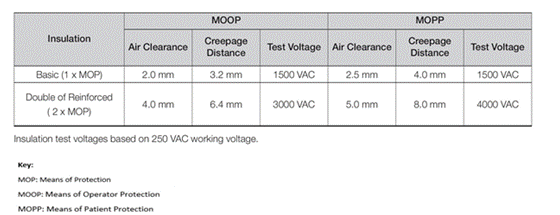
Table 1: Creepage, Clearance and Test Voltage for MOOPs and MOPPs
Leakage current must be limited
Touch current, patient auxiliary current, and leakage current must also be limited. The touch current must not exceed 100µA under normal conditions and 500µA in a single fault condition (SFC), effectively limiting the leakage current under normal conditions to 500µA. Table 2 summarizes the limits.

Table 2: Patient Auxiliary and Leakage Current Limits
Electromagnetic Compatibility (EMC)
Medical devices must also meet the EMC requirements of IEC 60601-1-2 (currently 4th edition). This latest version expands and severely limits the scope of immunity testing to include wireless communications equipment that may be in close proximity to life-critical equipment. Devices include cell phones and other devices that include Blue TootTM, Wi-Fi, Tetra, RFID or paging capabilities. Version 4 also includes requirements for risk analysis, recognizing that medical equipment may operate in a variety of settings, including professional healthcare, the home, and uncontrolled “special” areas such as ambulances in emergency situations. Equipment manufacturers must consider these possibilities, specify what basic product operation is required, and mitigate the effects at the appropriate level of immunity.
Power Options
In BF-&CF rated medical devices, the power system is a key factor in meeting insulation, leakage current and EMC requirements. In a home medical environment, Class II or “ungrounded” equipment is required and therefore has nothing to do with ground insulation. However, while complying with EMC standards, enclosure and patient leakage current limits must also be met, which can be difficult at power above 300W without a grounded chassis.
“Medically certified” power supplies are common, but most power supplies do not have 1 x MOPP output to ground insulation or low enough patient leakage current for BF/CF applications due to excessive insulation capacitance. To solve this problem simply and inexpensively, a medically isolated DC-DC converter can be used on the power output to provide power only to the patient connection Circuit, which is usually low power (Figure 1). DC-DC features extremely low coupling capacitance, total leakage in the μA range, and insulation ratings of 1 x or 2 x MOPPs. The choice of 1 or 2 MOPP depends on the equipment’s possible external signal input/output connections and its insulation rating against hazardous voltages or ground (known or unknown). For example a diagnostic port or an Ethernet connection.

Figure 1: Medical power system using secondary DC-DC.
The range of medically certified DC-DC converters can be found in products above 1W (Figure 2). Fully regulated parts are available for battery-operated devices that may be connected to chargers or include signal interfaces that require MOPs, or fixed input DC-DC converters for the lowest cost if regulated rails are already available.
Figure 2: XPPower Medical Certified DC-DC Converter C1W to 20W.
Multiple Output Solutions
If multiple AC-DC power supplies are used in a system to supply multiple power rails, their touch and leakage currents will increase, even if certified to the highest medical level, and the total current may exceed allowable limits. At low power, the same solution is to use a larger AC-DC and multiple connected medically certified DC-DC converters with ultra-low coupling capacitance. If the power requirements for this method are too high, the AC DC can be in a bare-board or U-shaped enclosure with multiple outputs to meet leakage and touch current limits, up to around 300W. At higher power levels, consider a medically certified modular AC-DC power supply such as XP power’s fleXPower series (Figure 3).
Figure 4: XP Power CMP250 Series: 250W Convection Cooled with Medical (BF) Approval
Patient-connected medical devices are a challenge for power system designers to comply with medical safety and EMC standards. However, there is also the option to use a fully compliant power supply or a combination of AC-DC and DC-DC converters, which can minimize system certification and product cost, and provide a low-risk, quick-to-market solution.